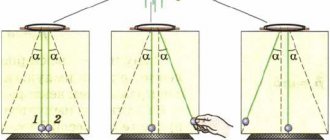
Operating principles of heat engines
Chapter 5. Thermal properties of gases, liquids and solids, gases and solids3. In a heat engine, only part of the energy that the working fluid receives from the heater is converted into mechanical energy. Part of the energy is transferred to the refrigerator, and part of the energy is used to perform work against resistance forces.
Let Q1 be the amount of heat received by the working fluid from the heater; Q2 is the amount of heat transferred to the refrigerator; A is the work done by the engine. Then A = Q1 - Q2.
| The ratio of the work A performed by the engine to the amount of heat received by it from the heater Q1 is called the heat engine efficiency factor (efficiency) η: |
The efficiency factor shows how much of the energy received from the heater was used to perform work. It is usually expressed as a percentage.
4. To increase the efficiency of a heat engine, it is necessary to increase the amount of heat it receives from the heater and reduce the amount of heat given to the refrigerator.
The higher its temperature, the greater the amount of heat received from the heater, and the lower the lower its temperature, the less the amount of heat given to the refrigerator. Therefore, to increase engine efficiency, the heater temperature should be increased and the refrigerator temperature reduced. In addition, to increase efficiency it is necessary to reduce energy losses through the walls of the engine and reduce friction in its working parts.
5. In heat engines, a certain amount of heat is transferred from the heater to the refrigerator and at the same time work is performed.
There are heat engines in which the reverse process is carried out: heat is transferred from the refrigerator to the heater, and in this case the work is done by external forces. Such heat engines are called refrigeration machines or refrigerators . A typical example of such machines is a household refrigerator.
In order to transfer a certain amount of heat from the refrigerator to the heater, it is necessary to expand the working fluid at a lower temperature than compression. The working fluid, expanding at the temperature of the refrigerator, receives from it the amount of heat Q2. External forces, producing work A, compress the working fluid at a higher temperature. In this case, the working fluid transfers the amount of heat Q1 = A + Q2 to the heater (Fig. 91). Thus, when the working fluid is compressed, energy is taken from the refrigerator and its temperature decreases. This, in particular, allows you to maintain low temperatures in the refrigerator and freezer compartments of a household refrigerator.
To perform work on the working fluid by external forces, energy is required. When operating a household refrigerator, electrical energy is consumed.
End >>>
GDZ Physics 8th grade. Humidity. Heat engine efficiency
Details Back to CONTENTS
What did Peryshkin keep silent about? About how to do your homework, answer questions and solve problems in the exercises! I am sure that thinking students will first do everything themselves, and this information will help those “stuck along the way.” Answers to PD in physics will help you test yourself and find mistakes. Answers to the DDs from the exercises correspond to all editions of this author’s textbooks, starting from 1989. Since the numbers of exercises with the same questions in different editions differ, the answers to the questions for the exercises are arranged according to the topics of the paragraphs. On this page, the DDZs are by topic: “Air humidity. Heat engine efficiency"
Go for it!
Project on thermal phenomena
- THERMAL PROCESSES
Thermal processes are a type of thermal phenomena; processes in which the temperature of bodies and substances changes, as well as a possible change in their states of aggregation.
Thermal processes include:
- Heating
- Cooling
- Vaporization
- Boiling
- Evaporation
- Crystallization
- Melting
- Condensation
- Combustion
- Sublimation
- Desublimation
Let us consider, as an example, a substance that can exist in three states of aggregation: water. Fig. 4. - in liquid - water, 0 < t < 100 0s>
Fig. 5. - in solid - ice, t < 0 0s
Fig. 6. - in gaseous - steam. t> 100 0s
Fig 4. Fig 5 Fig 6
- Heating
Heating is the process of increasing the temperature of a body or substance. Heating is accompanied by the absorption of heat from the environment. When heated, the state of aggregation of a substance does not change.
Formula for calculating the amount of heat when heating:
Q = cm(t2 - t1), where Q is the amount of heat,
c is the specific heat capacity of the substance, m is the mass of the substance,
t1 - initial temperature, t2 - final temperature,
Experiment 1: Heating.
Let's demonstrate heating experimentally.
Let's take water from the tap into a glass and measure its temperature (25°C), then put the glass in a warm place, and after a while measure the temperature of the water (30°C).
After waiting some more time, I measured the temperature again (35°C).
Fig 7 Fig 8 Fig 9
Conclusion: the thermometer shows an increase in temperature first by 5°C, and then by 10°C.
- Cooling
Cooling is the process of lowering the temperature of a substance or body; Cooling is accompanied by the release of heat into the environment. When cooled, the state of aggregation of a substance does not change.
Q = cm(t2 - t1), where Q is the amount of heat,
c is the specific heat capacity of the substance, m is the mass of the substance,
t1 - initial temperature, t2 - final temperature,
When cooling, Q becomes negative, since the final temperature becomes less than the initial one.
Experiment 2: Cooling.
Let's see how cooling occurs experimentally.
Let's take hot water from a tap into a glass and measure its temperature (60°C), then place this glass on the windowsill for a while, after which we measure the temperature of the water and it becomes equal (20°C).
Fig10 Fig 11
Conclusion: the water is cooling and the thermometer shows a decrease in temperature.
- Vaporization
Vaporization is the process of transition of a substance from a liquid to a gaseous state. During vaporization, energy (heat) is absorbed from the environment.
The amount of heat absorbed from the environment during vaporization is calculated by the formula:
Q = rm, where Q is the amount of heat, m is the mass of the substance,
r is the specific heat of vaporization.
Steam generation can be carried out in two ways:
evaporation and boiling.
- Boiling
Boiling is a process of intense vaporization in which vapor bubbles grow and rise up inside the liquid.
Boiling point is the temperature at which a substance begins to boil. This temperature is different for different substances.
During the boiling process, the temperature of the liquid does not change, that is, it remains constant.
The boiling point depends on the pressure exerted on the free surface of the liquid. When this pressure increases, the growth and rise of bubbles inside the liquid begins at a higher temperature, and when the pressure decreases, at a lower temperature. As the pressure decreases, the boiling point of water becomes less than 1000C. For example, in mountainous areas (at an altitude of 3 km, where the atmospheric pressure is 70 kPa, water boils at 900 C
Experiment 3: Boiling.
We encounter boiling water every day at home.
Pour water into the kettle and place it on the stove. First, the water heats up, and then the water boils. This is evidenced by the steam coming out of the kettle's spout.
Fig 12
Conclusion: when water boils, steam from the neck of the kettle comes out through a small hole and whistles, and we turn off the stove.
- Evaporation.
Evaporation is vaporization occurring from the free surface of a liquid.
Evaporation can occur from both closed and open surfaces of the liquid.
Evaporation depends on:
- Substance temperatures
(the higher the temperature, the more intense the evaporation);
- (the larger the area, the greater the evaporation);
- (different substances evaporate at different rates).
- (in the presence of wind, evaporation occurs faster);
Experiment 4: Evaporation.
We will observe evaporation experimentally.
If you have ever looked at lawns after rain, then you have undoubtedly noticed that the puddles become smaller and smaller. What happened to the water?
Conclusion: she evaporated!
- Crystallization
Crystallization (solidification) is the transition of a substance from a liquid state of aggregation to a solid state. Crystallization is accompanied by the release of energy (heat) into the environment.
The amount of heat released is calculated by the formula:
Q = λm, where λ is the specific heat of fusion, m is the mass of the body.
Liquid substances begin to crystallize at the temperature at which similar solid substances begin to melt. This temperature is called the crystallization (solidification) temperature.
During crystallization, the temperature also remains constant.
Experiment 5: Crystallization.
To detect crystallization, let's conduct an experiment.
Let's take water from the tap into a glass and put it in the freezer of the refrigerator. After some time, the substance hardens, i.e. a crust appears on the surface of the water. Then all the water in the glass completely turned into ice, that is, it crystallized.
Fig 13 Fig 14 Fig 15
Conclusion: first the water cools to 0 degrees, then freezes.
- Melting
Melting is the transition of a substance from a solid to a liquid state. This process is accompanied by the absorption of heat from the environment. To melt a solid crystalline body, a certain amount of heat must be transferred to it.
The amount of heat spent on melting the body is calculated by the formula:
Q = λm, where Q is the amount of heat, m is the mass of the body.
λ is the specific heat of fusion.
Each substance has its own specific melting point - the temperature at which the process of transition of a substance from solid to liquid begins. During melting, the temperature remains constant.
Experiment 6: Melting.
Melting is easily detected experimentally.
We take out a glass of frozen water from the freezer compartment of the refrigerator, which we placed. After some time, water appeared in the glass - the ice began to melt. After some time, all the ice melted, that is, it completely turned from solid to liquid.
Fig 16 Fig 17 Fig 18
Conclusion: Ice receives heat from the environment over time and will melt over time.
- Condensation.
Condensation is the transition of a substance from a gaseous state to a liquid state.
Condensation is accompanied by the release of heat into the environment.
The amount of heat released during condensation is calculated by the formula:
Q = rm, where Q is the amount of heat, m is the mass of the substance,
r is the specific heat of vaporization.
Experiment 7: Condensation.
Let us discover condensation experimentally.
We boiled water and held a cold mirror to the spout of the kettle. After a few minutes, drops of condensed water vapor are clearly visible on the mirror.
Fig 19 Fig 20
Conclusion: steam settling on the mirror turns into water.
The phenomenon of condensation can be observed in summer, in the early cool mornings. Droplets of water on grass and flowers - dew - indicate that the water vapor contained in the air has condensed.
- Combustion
Combustion is the process of burning fuel, accompanied by the release of energy. This energy is used in various areas of our lives.
The release of energy during combustion is explained by the fact that atoms combine into molecules without doing any work to overcome the forces of attraction between them.
The amount of heat released during combustion is calculated by the formula:
Q = qm, where Q is the amount of heat released, q is the specific heat of combustion of the fuel,
m is the mass of the substance.
Experiment 8: Combustion.
Every day we can watch natural gas burn in a stove burner. This is the process of fuel combustion. Also the process of fuel combustion is the process of burning wood.
Therefore, to conduct an experiment on fuel combustion, you just need to light a gas burner or a match.
Fig 21 Fig 22
Conclusion: when fuel burns, heat is released and a specific smell may appear.
- Sublimation.
Sublimation is the transition of a substance from a solid to a gaseous state (bypassing the liquid) from the Latin word “sublimo” - I exalt.
For example, graphite can be heated to two thousand degrees, nevertheless it will not turn into a liquid: it immediately turns from a solid state into a gaseous state.
- Desublimation.
Desublimation is the transition of a substance from a gaseous state to a solid state.
For example: the formation of patterns of crystalline ice on windows in winter. These beautiful patterns are the result of desublimation of water vapor in the air.
- CONCLUSION.
In my project work, I studied the most common thermal processes: heating, cooling, vaporization, boiling, evaporation, melting, crystallization, condensation, combustion, sublimation and desublimation.
In addition, the work covered such topics as heat transfer, methods of heat transfer, thermal motion, aggregate states of substances, as well as the general theory of thermal phenomena and thermal processes.
The basic formulas for calculating the amount of heat in various thermal processes were studied
Based on simple experiments, one or another thermal phenomenon was considered. The experiments are accompanied by demonstration pictures.
- CONCLUSIONS:
- The theory on the topic “Thermal Phenomena” has been fully studied;
- Based on eleven experiments, the existence of various thermal processes is considered;
- The relevance of thermal processes in human life has been proven.
- The goal and objectives of the project work that I set were completed.
Thank you for your attention!
- LITERATURE
- Physics textbook 8th grade (Peryshkin).
- Textbook of entertaining physics L.A. Gorev
- Physics textbook 8th grade. Shakhmaev N.M., Bunchuk A.V.