Covalent bond - definition, characteristics. What is a covalent bond?
The term “covalent bond” itself comes from two Latin words: “co” - together and “vales” - having force, since this is a bond that occurs due to a pair of electrons that belongs simultaneously to both
atoms (or in simpler terms, the bond between atoms due to a pair of electrons that are common to them). The formation of a covalent bond occurs exclusively among non-metal atoms, and it can appear both in the atoms of molecules and crystals.
For the first time covalent
The chemical bond was discovered back in 1916 by the American chemist J. Lewis and existed for some time in the form of a hypothesis, an idea, only then was it confirmed experimentally. What did chemists find out about it? And the fact that the electronegativity of non-metals can be quite large and during the chemical interaction of two atoms the transfer of electrons from one to another may be impossible, it is at this moment that the electrons of both atoms unite, a real covalent bond of atoms arises between them.
Chemistry lesson notes: Covalent polar chemical bond, grade 8
Summary of a chemistry lesson for 8th grade students on the topic “Covalent polar chemical bond”
Purpose: to organize students’ activities to study types of chemical bonds using the example of a covalent polar chemical bond Objectives: • Educational: to study the mechanism of formation of a covalent polar chemical bond; learn to compose electronic formulas of molecules of substances with covalent polar bonds; introduce the concept of electronegativity and, on the basis of this, explain the nature of a polar covalent bond. •Educators: create conditions for fostering activity and independence; continued education of a chemically literate individual; cultivate a culture of scientific work; increase interest in the problems of modern science. •Developmental: develop students’ memory and attention, ability to analyze; create positive motivation for studying the subject of chemistry; develop the ability to determine the type of covalent bond; learn to apply knowledge in a new situation; develop logical thinking and the ability to independently draw conclusions through generalizations. Equipment and reagents: periodic table of chemical elements by D. I. Mendeleev; textbook: Gabrielyan O. S. Chemistry. 8th grade; workbook; interactive board. Type of lesson: combined Form of organization of educational activities: - independent work with the text of the textbook, interactive whiteboard. — frontal; Planned results Subject
knowledge: • the mechanism of formation of covalent polar chemical bonds;
• concept of electronegativity Able to: • establish cause-and-effect relationships: composition of a substance - type of chemical bond - properties; • establish differences between the concepts of “covalent polar bond” and “covalent non-polar bond” • draw up schemes for the formation of molecules of compounds with a covalent polar chemical bond; • write and distinguish electronic and structural formulas of compounds Possess: • skills to independently use textbook materials and reference tables, apply previously acquired knowledge. Metasubject
Cognitive UUD: skills to reproduce information from memory;
work with various sources of information; compare and analyze information, draw conclusions; give definitions to concepts; express your thoughts freely and correctly in oral and written forms. Regulatory UUD: the ability to determine the degree of success in performing one’s work based on existing criteria; self-assessment and self-analysis skills. Communicative UUD: the ability to defend one’s point of view by arguing it. Personal
• demonstrate a responsible attitude to learning and show interest in the proposed activities, taking into account their own interests; • show respect for elders (teacher) and classmates and the results of their activities; • evaluate their activities, determining according to given criteria its success and ways to adjust it;
Download Chemistry lesson notes (8th grade) on the topic: “Covalent polar chemical bond”
We recommend watching:
Chemistry Extracurricular Activity for Grade 8 Chemistry Worksheet Grade 8 Chemistry Travel Lesson for Grade 8. Reasons Generalization of the section of the 8th grade chemistry course “Basic classes of inorganic substances.” Chemistry tournament
Similar articles:
Extracurricular activity in chemistry for 8th grade
Summary of a chemistry lesson in 8th grade “Salts. Grounds"
Extracurricular activity in chemistry in 8th grade
Types of chemical bonds. Covalent and ionic bond
As is known, atoms cannot exist in isolation from each other. They are part of either simple or complex substances.
Only noble or inert gases are monoatomic molecules. Other substances may contain two atoms, hundreds or even thousands of atoms. The force that binds these molecules, radicals or crystals
called
a chemical bond
.
Thus, the chemical bond
–
this is an interaction that connects individual atoms into more complex systems (molecules, radicals, crystals, etc.)
.
It is energetically unfavorable for atoms to exist in isolation from each other.
, therefore, when they interact with each other, a more
stable state
, that is, a state with the minimum possible energy reserve. This condition is the main reason for the formation of a chemical bond.
And the main condition for the formation of a chemical bond is a decrease in the total energy of the system compared to the total energy of isolated atoms.
For example, when atoms A and B interact, substance AB is formed; the energy of this substance will be less than the total energy of individual atoms A and B.
That is why the formation of a chemical bond is always accompanied by the release of energy
.
The nature of chemical bonding forces
–
electrostatic
, as it is caused by various types of interaction between positively charged nuclei and negatively charged electrons.
Valence electrons
take part in the formation of a chemical bond , that is, those electrons that are at the outer energy level and are least tightly bound to the nucleus. When a chemical bond is formed, each atom wants to complete its outer energy level.
An outer energy level is considered complete if it has 8 electrons, with the exception of the first period, where 2 electrons are needed to complete the outer level.
This state can be achieved if the atoms, when forming a chemical bond, combine their electrons to form a common electron pair.
Depending on the method of sharing electrons,
covalent
,
ionic
and
metallic bonds
.
Covalent bond
occurs between two nonmetal atoms with the same or different electronegativity values.
Let's consider the formation of a chemical bond using the example of a hydrogen molecule.
Each hydrogen atom has one electron in its outer energy level and is one electron short of completing the outer level. When two hydrogen atoms approach each other, partial overlap of electron clouds of unpaired electrons with antiparallel spins occurs. In the zone of cloud overlap, an area of increased electron density
.
The formation of this chemical bond can be shown using electronic formulas
, where valence electrons are shown as dots, or using graphic (structural) formulas, where a pair of electrons is indicated with a dash.
Electronic formulas
Each dash indicates a covalent bond.
. The formation of a chemical bond can also be shown using electron graphic diagrams, which indicate the orbitals of the outer energy level.
Graphic (structural) formulas
Thus, when a hydrogen molecule is formed, a chemical bond arises as a result of the overlap of two es orbitals.
Electronic graphic circuits
That is, a covalent bond
–
is a chemical bond that occurs as a result of the sharing of electrons to form common electron pairs
.
In a hydrogen molecule, the atoms are connected by one chemical bond. This type of bond is called a single bond.
Moreover, this covalent bond was formed by overlapping atomic orbitals along the bond line, therefore such a bond is called a sigma bond
(
Let's consider an example of the formation of chemical bonds in a nitrogen molecule.
The nitrogen atom has five electrons in its outer energy level; it lacks three electrons to complete the outer layer. Therefore, three unpaired electrons from each atom take part in the formation of a chemical bond. The formation of a nitrogen molecule can also be depicted in the form of an electronic and graphical formula.
Electronic formula
Graphic (structural) formula
The pair of electrons that forms a covalent bond
, is called
bonding
, and that
pair of electrons that does not participate in the formation of a bond
is called
nonbonding
. It is also called a lone pair of electrons, since it belongs to only one atom. Each nitrogen atom has one such pair of electrons.
In a nitrogen molecule, a triple bond occurs between two atoms.
Moreover, one bond was formed by overlapping PE-electron clouds along the communication line, therefore this is a sigma bond. The other two bonds were formed by overlapping vertically directed clouds of pe electrons.
This overlap no longer occurs along the line connecting the centers of the atoms, but on both sides of it. This creates two areas of overlap. This connection is called a pi bond
.
Sigma communication
–
it is a covalent bond that occurs when electron clouds on either side of a line connecting the nuclei of atoms overlap
.
Only pe and de clouds take part in the formation of pi bonds
.
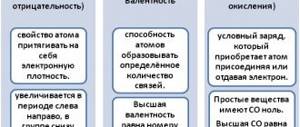
There are two types of covalent bonds
:
polar
and
non-polar
.
We looked at examples of the formation of hydrogen and nitrogen molecules; this covalent bond is formed by atoms of the same chemical element; an electron pair is symmetrically located between two atoms with the same electronegativity. Such a covalent bond is called nonpolar
.
Covalent polar bond
occurs between atoms of different chemical elements, that is, with different electronegativity, and the common electron pair is shifted to an atom with higher electronegativity.
For example, in the formation of a hydrogen chloride molecule, hydrogen and chlorine atoms take part, which differ in electronegativity, and the common electron pair will be shifted to the chlorine atom, because it is more electronegative than hydrogen.
When a molecule is formed, the es electron cloud of the hydrogen atom and the pe electron cloud of the chlorine atom overlap. , a partial negative charge appears on the chlorine atom
, and on the hydrogen atom there is
a partial positive charge
, which is conventionally denoted by the Greek letter “
delta
”, which shows that this charge is less than unity.
The charge value can be used as an estimate of bond polarity: the greater the partial charges on the atoms, the greater the bond polarity
. If we take a molecule of hydrogen fluoride and hydrogen chloride, then the bond in the hydrogen fluoride molecule will be more polar than in the hydrogen chloride molecule, since the partial charges on the hydrogen and fluorine atoms are plus zero point forty-three hundredths and minus zero point forty-three hundredths, and on the hydrogen and chlorine atoms – plus zero point eighteen hundredths and minus zero point eighteen hundredths.
Polar molecules can be represented as a dipole
, in which one pole is positive and the other is negative.
For example, the bond in the hydrogen chloride molecule is polar covalent
, and the molecule itself is also polar.
In the methane molecule the situation is different. Carbon-hydrogen bonds are polar, but the molecule itself is non-polar. This is explained by the fact that the methane molecule has the shape of a tetrahedron and the polarity of all bonds is mutually compensated.
Therefore, the polarity of a molecule depends on the polarity of the bonds and on the geometry of the molecule. Thus, a water molecule has an angular structure, therefore its molecule is polar and represents a dipole, and a carbon monoxide molecule has a linear structure, therefore the molecule itself is non-polar.
There are two main mechanisms for the formation of covalent bonds
– this is
exchange
and
donor-acceptor
.
For example, three unpaired electrons from the nitrogen atom and one electron from each hydrogen atom take part in the formation of an ammonia molecule. The nitrogen atom still has one more lone pair of electrons. Each bond between nitrogen and hydrogen is polar, so the entire ammonia molecule is a dipole, shaped like a pyramid with a nitrogen atom at the top.
Therefore, the mechanism of formation of a covalent bond due to the sharing of unpaired electrons of two interacting atoms is called exchange
.
In addition, the formation of a covalent bond is also possible through the interaction of atoms, one of which has a pair of unshared electrons, and the other has a free orbital. For example, during the formation of an AB molecule. In this case, atom A provides a pair of electrons to atom B, and this pair of electrons becomes bonding and a covalent bond occurs.
An atom that provides an electron pair
, is called
a donor
,
and an atom that has a free orbital
is called
an acceptor
.
Therefore, this mechanism of covalent bond formation is called
donor-acceptor .
Donor-acceptor mechanism of covalent bond formation
–
This is a mechanism in which a covalent bond occurs due to a lone pair of electrons of one atom and a free orbital of another atom
.
Let us analyze this mechanism using the example of the formation of ammonium ion. It is formed as a result of the interaction of ammonia with an acid solution.
The lone pair of nitrogen electrons and the free orbital of the hydrogen ion take part in the formation of a chemical bond in the ammonium ion.
The donor-acceptor mechanism explains the existence of the hydronium ion; this particle is formed as a result of the hydration of the hydrogen ion. When a hydronium ion is formed, the electron pair donor is oxygen, and the acceptor is a hydrogen ion, which provides a free orbital.
Covalent bonds have their own characteristics. One of the important characteristics of a covalent bond is its strength.
.
A measure of this strength is the energy that must be expended to break a chemical bond
.
This characteristic is called binding energy
.
For example, a hydrogen molecule has a binding energy of 435 kilojoules per mole, a fluorine molecule has 159 kilojoules per mole, and a nitrogen molecule has 943 kilojoules per mole. Accordingly, the lower the binding energy, the less strong the covalent bond and the greater the reactivity of the substance.
Another important characteristic of a covalent bond
is
the bond length
, that is,
the distance between the nuclei of atoms
.
As atomic radii increase, bond length
between them increases, and
the strength of the connection
decreases
.
For example, the bond between hydrogen atoms is stronger than the bond between fluorine atoms, so the length of its bond is zero point seventy-four thousandths of a nanometer, and the bonds between fluorine atoms are zero point one hundred and forty-two thousandths of a nanometer. For example, in organic compounds, the single bond length is zero point one hundred and fifty-four nanometers, the bond energy is 348 kilojoules per mole, the double bond length is zero point one hundred thirty-three nanometers, the bond energy is 635 kilojoules per mole, and the triple bond length is zero point one hundred twenty nanometers, energy this bond is 830 kilojoules per mole. Thus, the energy of a double or triple bond is less than double or triple the energy of a single bond, so a single bond
, which is a sigma bond,
stronger than the pi bond.
A covalent bond is characterized by saturation
. That is, the number of covalent bonds that an atom can form is limited. The number of bonds that a particular atom can form is determined by the number of orbitals that take part in the formation of a chemical bond.
For example, elements of the second period, which have only 4 orbitals at the outer level (one es and three pe orbitals) can form no more than 4 covalent bonds. De-orbitals of the external and pre-external energy levels also take part in the formation of chemical bonds in other atoms.
A covalent bond is characterized by direction
, since electron clouds of various shapes take part in the formation of this connection, and they are located in space so that their overlap is maximum.
If ES clouds overlap
, then the covalent bond can be located in any direction relative to the center of the atom
. If the covalent bond is formed due to the overlap of the PE clouds
, then
the area of overlap is located along the bond line and is determined by the spatial orientation of the PE cloud
.
Let's consider an ionic bond. It occurs between atoms with different electronegativity.
Moreover, in contrast to a covalent polar bond, the difference in the electronegativity of the atoms should be large
, so
the shared electron pair is almost entirely shifted towards the atom with higher electronegativity
. As a result, positively and negatively charged ions are formed. These ions are held together by electrostatic attractive forces.
Thus, the ionic bond
–
this is a chemical bond that occurs due to the electrostatic interaction of oppositely charged ions
.
Typically, ionic bonds form between atoms of typical metals and typical nonmetals
. For example, sodium chloride. A sodium ion is formed when one electron is removed from an atom, and a chlorine ion is formed when one electron is added to a chlorine atom. Electrostatic attraction occurs between these resulting ions, resulting in the formation of an ionic compound.
When a chemical bond was formed, electrons from the sodium atom passed to the chlorine atom and oppositely charged ions were formed, which have a completed external energy level.
It has been established that electrons from the metal atom are not completely detached, but are only shifted towards the chlorine atom, as in a covalent bond
.
And this shift is greater, the greater the difference in electronegativity
. A typical example of an ionic bond is cesium fluoride, where the difference in electronegativity is very large, but even here the electron of the cesium atom is not completely transferred to the fluorine atom.
Therefore, we can talk about an ionic chemical bond with a certain proportion of covalent bond.
Most binary compounds that contain metal atoms are ionic. For example, oxides, halides, sulfides, nitrides.
Ionic bonding also occurs between simple cations and simple anions, as well as between simple cations and complex anions. Therefore, ionic compounds include salts and bases.