Chemistry lesson “Foundations” /8th grade/
Municipal budgetary educational institution
«Zinovskaya secondary school"
Shkolny lane, 2, Zinovo village, Yalutorovsky district, Tyumen region, 627031
tel. 8(34535)99117, tel/fax: 8(34535)99183, e-mail: [email protected]
Vorobyova Lyudmila Valerievna,
chemistry teacher,
first qualifying
category
Topic of chemistry lesson in 8th grade: “FOUNDATIONS”
The purpose of the lesson
: introduce students to a new class of inorganic compounds - bases.
Lesson Objectives
:
- Educational: systematize students’ knowledge about oxides; introduce
with bases; study classification and physical properties; teach to recognize these grounds; introduce the use of principles in life
- Developmental: strengthen the skills of compare, contrast, analyze, draw conclusions
- Educational: to help students develop the ability to work in a team; develop accuracy in students
Equipment
: cards “Signs of chemical elements”; crossword; cards with table, multimedia set
During the classes:
- Organizing time.
“Soft landing” (name the signs of chemical elements)
- Checking homework
Make up formulas for the oxides named in the text:
In the earth's crust - the lithosphere - there are aluminum oxide ____ (clay), silicon oxide (IV) ___ (sand), iron oxide (III) ___ (found in red iron ore).
The water shell of the Earth - the hydrosphere - is hydrogen oxide _____.
There is carbon monoxide (IV) ____ (carbon dioxide) in the air.
As a result of human economic activities, substances are formed that pollute the atmosphere: carbon monoxide (II) _________ (carbon monoxide), sulfur oxide (IV) _____ (sulfur dioxide), nitrogen oxide (II) _________ and nitrogen oxide (IV)__________.
Peer review
(answers and a rating scale are written on the board:
Al 2 O 3 , SiO 2 , Fe 2 O 3 , H 2 O , CO 2 , CO , SO 2 , NO , NO 2.
9 points - “5”, 7-8 points – “4”, 6-4 points – “3”, 3 or less points – “2”)
- Communicating the theme and goals of rock
Formulas of substances are written on the board; students are asked to read and name the formulas they know:
Fe2O3, CuO, ZnO, NaOH, N2O5, H2O, Mg(OH)2, Li2O, Fe(OH)3, CaO, K2O, BaO, Ba(OH)2, SO3, Fe(OH)2
- What class of inorganic substances do they belong to? How to call it in one word?
— Can we say that these are binary compounds? Why?
- What do you call other substances?
— To find out, we need to solve a crossword puzzle and in the highlighted cells you will read their names.
1. Chemical element or coniferous forest (BOR)
2. A chemical element whose nuclear charge is +47. (SILVER)
3. A chemical element whose etymology is associated with a geographical name (country name) (GERMANIUM)
4. Complex neutral particle consisting of protons, electrons, neutrons (ATOM)
5. The most common oxide on Earth (WATER)
6. A nonmetal whose relative atomic mass is 14. (NITROGEN)
7. A metal that has 11 electrons, 11 protons and 12 neutrons in an atom (SODIUM)
8. Positive or negative particle (ION)
9. Chemical electron, the name of which consists of the names of two animals (ARSENIC)
— What are the names of our substances? (BASES)
— Thus, the topic of our lesson is ______ (“Fundamentals”)
— What goal will we set for ourselves? (fixed on the board)
— And to achieve our goal, what tasks will we set? (children's answers are recorded on the board)
- Learning new material
1. Derivation of the definition.
- To solve our first problem, we need to compare our substances. How are they different and what are their similarities? (children's answers)
- Thus, we conclude that grounds are... (a definition is drawn together with the children)
- Let's compare our definition with the definition in the textbook on p. 98.
(children read it, then write it down in dictionaries)
- What is the general formula of grounds? (M(OH)p, where M is a metal, and p is the number of OH- groups.
— The OH- ion is what kind of ion: simple or complex? Why?
— Positive or negative? Why? (proved through the charge of simple ions).
2. Reading and naming the bases.
- How will we read the formulas?
- Read the formulas of our substances.
- Now give them a name. To find out how to name the grounds, find and read paragraph 19 in the text.
- Name our substances.
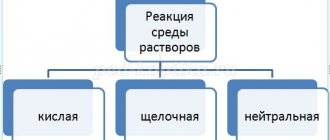
3. Classification of bases (relative to water)
- What two groups can bases be divided into in relation to water? (Soluble, insoluble)
- How to find out? (According to the solubility table)
- Soluble bases are called ALKALI (vocabulary work)
— Make a diagram in your notebooks “Classification of bases” (check in pairs)
— Give examples of soluble bases. Write them down.
- Insoluble bases. Write them down.
PHYSMINUTE
4. Acquaintance with some representatives of the foundations.
Work in groups. Filling out the table
| Base name | Chemical formula | Trivial name | Physical properties | Application | Note |
— Table check
- Reinforcing the material learned
— Why are sodium and potassium hydroxides called caustic alkalis? (because they are very caustic, i.e. they corrode paper, leather, fabrics and other materials)
— Make up formulas for the following bases: zinc hydroxide, aluminum hydroxide, copper (II) hydroxide, copper (I) hydroxide, lithium hydroxide.
- Make up formulas of oxides corresponding to our bases and give them names ( ZnO ,
Al 2 O 3 , CuO , Cu 2 O, Li 2 O )
- Calculate the mass fractions of the elements oxygen (1 row) and hydrogen (2 row) in sodium hydroxide .
| Given : NaOH ___________ Find : w (O2)- ? w(H2) — ? | Solution : 1. Mass fractions of elements in a substance are calculated using the formula: w (E) = n*Ar(E) Mg(v-va) 2. Find the relative molecular mass of the substance (sodium hydroxide): Mg (NaOH) = 23+16+1=40 3. Calculate the mass fractions of the oxygen element (row 1) in sodium hydroxide: w (O2) =1*16/40=0.4, or 40% 4. Calculate the mass fractions of the element hydrogen (row 2) in sodium hydroxide: w(H2) = 1*1/40=0.025, or 2.5% Answer : w (O2) =0.4, or 40%; w(H2) = 0.025, or 2.5% |
- Summing up the lesson
- Remember what goal and what tasks of the lesson we set for ourselves? Have we achieved the goal of the lesson? (children's answers)
— Have all the objectives of the lesson been achieved? Have you forgotten anything? (children's answers)
- Evaluate your work in class. Give yourself a grade in the margins of your notebooks, leave your notebooks on your desks.
- Homework
— Paragraph 19, repeat definitions, chemical symbols, formulas, solve problems 4 or 5 on p. 102 (optional) and No. 3, p. 101
Annex 1
BASES
| Base name | Chemical formula | Trivial name | Physical properties | Application | Note |
| Sodium hydroxide | NaOH | Sodium hydroxide | A solid, white substance, hygroscopic, dissolves well in water, and heat is released. The solution in water is soapy to the touch and very caustic. | In the soap, leather and pharmaceutical industries | This hydroxide and its solutions must be handled carefully, being careful that they do not get on clothes and especially on the hands and face; wounds that take a long time to heal form on the skin from this substance |
| Potassium hydroxide | CON | Caustic potassium | A solid white substance that is highly soluble in water and produces heat. The solution in water is soapy to the touch and very caustic. | As an additive in the production of soap, refractory glass | |
| Calcium hydroxide | Ca(OH)2 | Slaked lime Transparent solution of Ca(OH)2 - lime water | Friable, white powder, slightly soluble in water | In construction for masonry and plastering of walls, for whitewashing trees, for producing bleach, which is a disinfectant | It is obtained by reacting quicklime CaO with water. This process is called quenching |
| Base name | Chemical formula | Trivial name | Physical properties | Application | Note |