Fats and lipoids
Fats are compounds of high-molecular fatty acids and trihydric alcohol glycerol. Fats do not dissolve in water - they are hydrophobic. There are always other complex hydrophobic fat-like substances called lipoids in the cell. One of the main functions of fats is energy. During the breakdown of 1 g of fat into CO2 and H2O, a large amount of energy is released - 38.9 kJ (~9.3 kcal). The fat content in the cell ranges from 5 to 15% of the dry matter mass. In living tissue cells, the amount of fat increases to 90%. The main function of fats in the animal (and partly plant) world is storage.
When 1 g of fat is completely oxidized (to carbon dioxide and water), about 9 kcal of energy is released. (1 kcal = 1000 cal; calorie (cal) is an extra-system unit of the amount of work and energy, equal to the amount of heat required to heat 1 ml of water by 1 °C at standard atmospheric pressure 101.325 kPa; 1 kcal = 4.19 kJ) . When 1 g of proteins or carbohydrates is oxidized (in the body), only about 4 kcal/g is released. In a variety of aquatic organisms—from single-celled diatoms to basking sharks—fat will “float,” reducing average body density. The density of animal fats is about 0.91 – 0.95 g/cm³. The density of vertebrate bone tissue is close to 1.7 – 1.8 g/cm³, and the average density of most other tissues is close to 1 g/cm³. It is clear that you need quite a lot of fat to “balance” a heavy skeleton.
Fats and lipids also perform a construction function: they are part of cell membranes. Due to poor thermal conductivity, fat is capable of a protective function. In some animals (seals, whales) it is deposited in the subcutaneous adipose tissue, forming a layer up to 1 m thick. The formation of some lipoids precedes the synthesis of a number of hormones. Consequently, these substances also have the function of regulating metabolic processes.
Now we will do several Unified State Examination tasks in biology on the topic “Chemical composition of the cell”
Chemical composition of the cell
As you already know, all living organisms are made up of cells. And all cells are made of chemical elements.
In high school chemistry classes, you will become familiar with all the known chemical elements and the periodic table, which was invented in 1869 by the Russian chemist Dmitry Ivanovich Mendeleev.
Today we will introduce you to only some of the substances that form the basis of living organisms.
The earth's crust, for example, is half oxygen.
Oxygen
is a simple substance that, under normal conditions, is a colorless, tasteless, and odorless gas.
A quarter of the earth's crust is made of silicon
.
It is denoted by the symbol Si (sil and
cium). Silicon is part of sand.
The earth's crust also contains aluminum.
- light silver-white metal,
iron
,
calcium
- alkaline earth metal - and other elements.
And all living organisms are 98% composed of four basic elements - carbon
,
oxygen, hydrogen
and
nitrogen.
The relative content of these chemical elements in living matter is much higher than, for example, in the earth's crust.
As we see, both living organisms and inanimate nature contain the same chemical elements, such as oxygen. By the way, a person weighing 70 kg has up to 40 kg of oxygen. That is, more than half the body.
So, we said that all living organisms are 98% composed of four main elements: carbon, oxygen, hydrogen and nitrogen.
The remaining 2% of the cell mass comes from the following elements: potassium, sodium, calcium, chlorine, magnesium, iron, phosphorus
and
sulfur.
Other chemical elements (for example, zinc and iodine) are contained in very small quantities.
About 80 chemical elements have been found in the cells of living beings. The ratio of chemical elements in living and inanimate nature is different.
If we take a closer look at the chemical composition of living organisms, we will find that different organisms and even cells that perform different functions can differ significantly from each other. For example, buttercups accumulate lithium, duckweed - radium, and algae - an element necessary for the normal functioning of our thyroid gland - iodine.
Let's talk about the biological role of some chemical elements.
Hydrogen, oxygen, carbon, nitrogen are the basis of the organic substances of the cell.
Hydrogen
as a separate element it has no biological value. The compounds that it contains are important for the body, namely: water, proteins, fats, carbohydrates, etc. The greatest value, of course, is the combination of hydrogen with oxygen - water, which is the environment for the existence of all cells of the body.
K and oxygen
participates in many reactions of the body. Forms the “wet” substance of the cell - water.
Carbon -
the most important chemical element in the body.
Due to its unique chemical properties, it forms the chemical basis of life. Forming bonds with other atoms, it makes up the skeleton of organic molecules of different chemical composition, structure, length and shape.
Nitrogen
- a simple substance, a gas without color, taste or smell. One of the most common elements on Earth.
Pure nitrogen itself does not have any biological role. It performs its main functions as part of compounds: as part of proteins (important components of all living organisms), as part of DNA (through which all information is transmitted within the cell and by inheritance), and also as part of hemoglobin in the blood. Hemoglobin is a protein that binds to oxygen and ensures its transport through the bloodstream.
Calcium -
soft metal of silvery white color. It is part of bones and teeth. Participates in nervous and muscle activity, blood clotting. In plants it is part of the cell wall.
Phosphorus
It is also part of organic substances - mainly proteins and nucleic acid (DNA). In addition, phosphorus is an essential component of bones and tooth enamel.
Potassium
is part of all cells, ensures the conduction of nerve impulses, as well as the regulation of cardiac activity.
Sodium
often works together with potassium. And together with chlorine, sodium maintains osmotic pressure in the cell, which makes it strong and elastic.
Magnesium
is part of chlorophyll, necessary for photosynthesis processes.
Photosynthesis is the process of converting solar energy into the energy of chemical bonds of organic substances with the participation of photosynthetic pigments (chloroplasts). This process is carried out by green plants.
Iron
We will find in the composition of many enzymes that accelerate chemical reactions in the body. Iron is also found in blood hemoglobin.
About a quarter of inanimate nature consists of silicon
. It is involved in the formation of bones and is also part of the cell wall of plants.
Zinc
participates in the process that regulates blood sugar.
Copper
in some invertebrate animals (arthropods, mollusks) it turns the liquid, which performs the same functions as blood, blue.
Fluorine
part of tooth enamel. In our body it accumulates in bone tissue.
Iodine
needed for normal functioning of the thyroid gland.
Cobalt
- takes part in hematopoietic processes.
As we see, each chemical element plays its role in living organisms. Some functions require a high content of a certain element, while others require a low content. But absolutely all of them are vital.
Chemical elements combine with each other to form inorganic
and
organic
matter.
Inorganic substances of the cell
― this is water and mineral salts. The most important inorganic substance is water. Most water is contained in cells (from 40 to 95% of the total cell mass). Water gives the cell elasticity, determines its shape, and participates in metabolism.
Approximately 1–1.5% of the total cell mass is made up of mineral salts, in particular salts of calcium, potassium, phosphorus, etc.
And compounds of nitrogen, phosphorus, calcium and other inorganic substances are used for the synthesis of organic molecules (proteins, nucleic acids, etc.)
Organic matter
are found in all living organisms. These include carbohydrates, proteins, fats, nucleic acids and other substances.
Squirrels
play a vital role in cell life. They are part of various cellular structures and regulate vital processes.
Proteins transport important substances throughout the body. For example, hemoglobin
carries oxygen from the lungs to the cells of other tissues.
Specific proteins perform a protective function. They protect the body from invasion of foreign organisms and from damage. For example, the immune system reacts to the penetration of foreign proteins into the body.
Proteins are also a source of energy; when they are broken down, it is released.
Carbohydrates
- this is an important group of organic substances, as a result of their breakdown, which cells also receive the energy necessary for their life. Carbohydrates are part of cell membranes in the form of cellulose, giving them strength.
Storage substances in cells - starch and sugars - also belong to carbohydrates.
Fats
deposited in cells. When they are broken down, the energy needed by living organisms is also released.
Unified State Exam assignments on the topic
Exercise 1.
What structural components are included in the nucleotides of a DNA molecule?
Options:
- nitrogenous bases: A, T, G, C
- variety of amino acids
- lipoproteins
- carbohydrate deoxyribose
- Nitric acid
- phosphoric acid
Answer: 146
Solution: nucleotides are linked to each other through sugar (deoxyribose) and a phosphoric acid residue, and RNA nucleotides differ from DNA nucleotides in the structure of the carbohydrate they contain (ribose instead of deoxyribose), but they ask us about DNA, which means we also note deoxyribose.
Task 2.
What are the properties, structure and functions of polysaccharides in the cell?
Options:
- perform structural and storage functions
- perform catalytic and transport functions
- consist of monosaccharide molecule residues
- consist of amino acid residues
- dissolve in water
- do not dissolve in water
Answer: 136
Solution: Monosaccharides dissolve in water, but polysaccharides do not dissolve in water.
Task 3.
Which cellular structures contain circular DNA?
Options:
- ribosomal subunits
- nuclear chromosomes
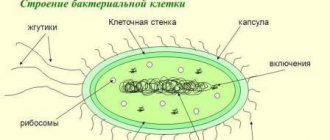
- bacterial nucleoids
- microtubules of the cytoskeleton
- chloroplasts
- mitochondria
Answer: 356
Solution: Human DNA, located in the nuclei of cells, has the shape of two helices connected to each other. DNA, which is found in other structures and organelles, and bacterial nucleoids are circular in shape.
Task 4.
What functions does water perform in a cell?
Options:
- construction
- solvent
- catalytic
- storing
- transport
- gives the cell elasticity
Answer: 256
Solution: Many substances are soluble in water, and hydrophobic structures can move throughout the body with the blood and lymph flow.
Task 5.
What function do nucleic acids perform in a cell?
Options:
- are keepers of hereditary information
- carry out homeostasis
- transfer hereditary information from the nucleus to the ribosome
- participate in protein synthesis
- are part of the cell membrane
- perform a signaling function
Answer: 134
Solution: Nucleic acids include DNA, mRNA, tRNA, rRNA. Each of these structures has its own function. Thanks to nucleic acids, processes such as DNA replication, translation and transcription occur.
Task 6.
Select RNA features.
Options:
- found in ribosomes and nucleolus
- capable of replication
- consists of one chain
- contained in chromosomes
- ATGC nucleotide set
- AGCU nucleotide set
Answer: 136
Solution: Nucleic acids RNA and DNA differ in the set of nitrogenous nucleotide bases, they contain different carbohydrates, and they also have different spatial shapes.
Task 7.
What functions do carbohydrates perform in the animal body?
Options:
- catalytic
- structural
- storing
- hormonal
- contractile
- energy
Answer: 236
Solution: Only enzymes that are proteins can be catalysts, and the hormonal function is inherent in lipids, because they are precursors of steroid hormones.
Task 8.
What features are characteristic of a DNA molecule?
Options:
- consists of one polypeptide strand
- consists of two polynucleotide strands twisted into a helix
- has a nucleotide containing uracil
- has a nucleotide containing thymine
- stores hereditary information
- transfers information about protein structure from the nucleus to the ribosome
Answer: 245
Solution: Nucleic acids RNA and DNA differ in the set of nitrogenous nucleotide bases: for DNA these are A, T, C, G, and for RNA - A, U, C, and G, moreover, they have different spatial shapes and perform different functions.
Task 9.
Monosaccharides in the cell perform the following functions:
Options:
- energy
- constituent components of polymers
- informational
- constituent components of nucleic acids
- protective
- transport
Answer: 124
Solution: When 1 g of glucose is broken down, 17 kJ of energy is released, and ribose and deoxyribose are part of RNA and DNA, respectively.
Task 10.
Lipids in the cell perform the following functions:
Options:
- storing
- regulatory
- transport
- enzymatic
- carrier of hereditary information
Answer: 12
Solution: Lipids are precursors of hormones that affect the functioning of the body.
Task 11.
What are the structural features and properties of protein molecules?
Options:
- have primary, secondary, tertiary, quaternary structures
- look like a single spiral
- monomers - amino acids
- monomers - nucleotides
- capable of replication
- capable of denaturation
Answer: 136
Solution: In the body, proteins are most often found in the form of globules. At high temperatures or exposure to radiation, the protein structure can be destroyed.
Task 12.
Proteins and lipids play a role in the formation of:
Options:
- ribosomes
- membranes of mitochondria and chloroplasts
- plasma membrane
- kernel shell
- microtubules
- centrioles
Answer: 234
Solution: The membranes of all cell organelles are similar in structure to the cytoplasmic membrane.
Task 13
What are the characteristics of enzymes?
Options:
- are fragments of a DNA molecule
- have a protein nature
- speed up chemical reactions
- participate in thermoregulation
- regulate life processes
- may contain vitamins
Answer: 124
Solution: Enzymes in the body perform a catalytic function.
Task 14.
What functions do lipids perform in the animal body?
Options:
- enzymatic
- storing
- energy
- structural
- contractile
- receptor
Answer: 234
Solution: Lipids are part of the cell membrane, and when 1 g of fat is burned, 39 kJ of energy is released
Task 15.
Select the structural features of protein molecules.
Options:
- composed of fatty acids
- consist of amino acids
- monomers of the molecule are held together by peptide bonds
- consist of monomers of identical structure
- are polyhydric alcohols
- the quaternary structure of molecules consists of several globules
Answer: 236
Solution: Protein molecules are made of amino acids held together by peptide bonds. There are 20 amino acids in the human body, 8 of which are essential.
Task 16.
All of the signs below, except two, can be used to describe the significance of proteins in the human and animal body. Identify two characteristics that “drop out” from the general list, and write down the numbers under which they are indicated in your answer.
Options:
- serve as the main building material
- are broken down in the intestines to glycerol and fatty acids
- formed from amino acids
- Converted to glycogen in the liver
- act as enzymes to speed up chemical reactions
Answer: 24
Solution: Glycogen is synthesized in the liver under the influence of the hormone insulin, secreted by the pancreas, from glucose when its content in the blood is elevated.
Task 17.
All but two of the following features can be used to determine the functions of lipids in a cell. Identify two characteristics that “drop out” from the general list and write down the numbers under which they are indicated in the table.
Options:
- storing
- regulatory
- transport
- enzymatic
- construction
Answer: 34
Solution: Lipids are part of cell membranes, perform a storage function and are precursors of hormones.
Task 18.
All but two of the following characteristics can be used to describe egg white albumin. Identify two characteristics that “drop out” from the general list and write down the numbers under which they are indicated in the table.
Options:
- consists of amino acids
- digestive enzyme
- denatures reversibly when eggs are cooked
- monomers are linked by peptide bonds
- the molecule forms primary, secondary and tertiary structures
Answer: 23
Solution: At very high temperatures, the primary structure of the protein is destroyed.
Now you know what the chemical composition of a cell is, you can answer some of the exam questions. The main thing is to clearly learn the composition of the cell and what each of its components does in the body.