What does everything consist of? Molecules
Now bodies are examined using very powerful electron microscopes. Through them it is clear that the substance is represented by many tiny moving “bricks”. These “building blocks” are different for different substances. They are called molecules. And if you imagine that your eyes are “microscope eyes,” then you can mentally see these small particles.
Molecules, it turns out, consist of atoms, and they are even smaller particles. For example, oxygen and hydrogen atoms combine to form the famous water molecule. There is a humorous phrase: “My boots let H2O through.” H2O is the chemical formula of the water molecule. H – designation of hydrogen, O – oxygen. Index 2 in this case denotes the number of hydrogen atoms in the water molecule. The molecules of hydrogen and oxygen gases are already new. They contain two hydrogen and oxygen atoms (H2 and O2).
()
There are substances consisting only of atoms. These are metals and inert gases (for example, neon in advertising illumination). Different combinations of atoms give rise to new substances that exist in nature.
People have long assumed that matter is made up of molecules and atoms. Back in the fifth century BC, the Ancient Greek scientist Democritus expressed such a hypothesis. In Russia, the doctrine of the molecular structure of matter was developed by M.V. Lomonosov in the eighteenth century.
How to estimate the sizes of mysterious small particles?
Following the rule of measurement, they need to be compared with something. This thought experiment is interesting: a seventh grader and a molecule. If the molecule is enlarged several times so that it becomes the size of a seventh grader, but also enlarged by the same number of times, then the seventh grader can reach the Sun. Science, of course, does not use such comparisons. Modern instruments - microscopes - make it possible to determine the sizes of atoms and molecules quite accurately. It is also possible to count the number of small particles in any body.
It is easy to guess that the number of molecules of the substance is huge. And again, an interesting comparison: if there were as many people on planet Earth as there are molecules in one cubic centimeter of air, then five thousand people could fit on one square kilometer of Earth.
So, matter consists of molecules, and molecules of atoms;
- different substances have different molecules (combinations of atoms) in structure;
- molecules are microscopically small, but there are a huge number of them in the body.
Structure of matter. Molecules
Structure of matter
You cannot know existence from the outside, you can only know it from within.
Nikolay Berdyaev
This topic is devoted to the structure of matter. Even in ancient times, people suggested that all substances consist of very small particles
. However, only in the eighteenth century did these assumptions develop into a more or less coherent theory. Ideas about the structure of matter have helped people not only understand and explain certain physical phenomena, but also influence the origin of phenomena and predict the behavior of various substances when external conditions change. Moreover, people have learned to produce substances with desired properties themselves.
Some phenomena can be easily predicted based on your everyday experience. For example, if you squeeze a balloon with your hands, it will change its volume and shape.
If you untie the balloon, the air will come out of it. If you drop a drop of paint into water, the water will become colored. If you heat ice, it will melt and turn into water.
Let us consider several targeted experiments that will provide some information about the structure of matter. Let's take the most ordinary leaf from a tree. Can it be divided? Of course, you can easily break it. The resulting pieces can be torn apart again and again. If you rub a small piece of a leaf with your fingers, you can see small particles remaining on your fingers. You can conduct a similar experiment with water. If you take a bottle containing a certain amount of water.
You can easily pour some of the water into another container, and from this container into another, and so on. Experiments like this tell us that a wide variety of substances are made up of particles.
By squeezing the ball, you can reduce its volume. It should be noted that the number of air particles inside the ball has not changed
.
Consequently, the distance between particles decreased
.
Similarly, you can stretch the rubber cord, thereby increasing its volume
.
Again, this does not change the number of particles.
Consequently, the distance between particles increases.
Thus, matter consists of particles, and between these particles there are certain spaces. But is it possible to reduce or increase these gaps only through mechanical efforts?
It turns out that
no
. You can carry out the following experiment: let's pass a ball suspended on a chain through a ring.
Let's heat this ball and try to pull it back out. After heating, the ball does not fit into the ring. This suggests that when heated, bodies expand, that is, their volume increases.
If you wait until the ball cools down, it will be able to pass through the ring again.
This means that when cooled, the volume of the body decreases. The same will happen with liquid.
Fill the vessel with water to the brim and seal it with a stopper.
We will insert a small glass tube into the cork. When heated, the water will partially fill the tube, which means that the volume of water also increases when heated.
This is well confirmed by another everyday experience:
if you lower a thermometer into a vessel with hot water, the column of mercury will creep up.
That is,
when mercury is heated, the spaces between its particles increase, which leads to an increase in volume.
Thus, when the particles of a body move away from each other, the volume of the body increases
.
Conversely, when the particles of a body move closer to each other, the volume of the body decreases.
The question may arise:
what kind of particles are we talking about if all bodies are solid and can be moved as a whole without worrying that these particles will crumble?!
More detailed explanations of these issues will be revealed with further study of physics. However, it is possible to carry out a fairly simple experiment to confirm that a substance consists of particles. Let's take three glasses of water and color the water in one of them.
Transfer some of the colored water to another glass. It can be seen that the water in this glass has also become colored, but the color is less saturated. If you then transfer some of the water from the second glass to the third, the water in it will also color, but only slightly. This experiment confirms that matter consists of particles.
The decreasing color saturation in glasses is explained by the presence of fewer paint particles.
And this, of course, is just one of many experiments confirming that substances consist of tiny particles.
Such particles were called molecules
.
Molecule translated from Latin means “ small mass
”.
The molecules are so small that the human eye is simply unable to see individual molecules.
To get an idea of how small molecules are, here are a few examples.
For example, an apple contains approximately the same number of molecules as apples could be packed inside our planet, the Earth. Another example: one drop of water contains approximately the same number of molecules as there are drops in the Black Sea. A very small volume of air - one cubic millimeter contains 27 × 1015, that is, millions of billions of molecules. Of course, we are not able to see such small particles without special instruments. One of these devices is called an electron microscope: with its help, we can see molecules and obtain images of them.
For example, the figure shows one type of blood particle - red blood cells, as well as vitamin C.
To date, scientists have studied many different molecules and can say with confidence that the molecules of different substances differ from each other, but the molecules of identical substances are absolutely the same.
Thus, water molecules can only consist of water and nothing else.
However, even molecules are not the smallest particles
.
They are made of atoms
(
atom means “indivisible” in Greek
). With further study of physics, it will be shown that the atom also has an internal structure. Molecules are usually depicted schematically: here, for example, is a schematic representation of a water molecule: it consists of two hydrogen atoms and one oxygen atom.
Each substance has its own designation.
For example,
hydrogen is denoted by the Latin letter H , and oxygen by the letter
O.
Thus, a water molecule has the chemical formula H 2 O - this formula shows that there are two hydrogen atoms and only one oxygen atom in the molecule.
If molecules are made up of atoms, why are molecules considered the smallest particles of a given substance?
The point here is this: molecules form matter the way we see it, touch it, feel it, and so on.
Main conclusions:
– All bodies are made of particles
.
– There are gaps between these particles.
– Dimensions
These particles
are very, very small
.
– Smallest particles
of this substance are called
molecules
.
– Molecules
consist of
atoms
.
– When heated
bodies
expand
, and when
cooled
,
they contract
.
Molecular movement
Will tea in a glass be sweet if the sugar is not stirred in it? Yes, sure. But after a certain time, during which sugar molecules from the bottom of the glass will rise to the upper layers of the liquid. This means the molecules are moving. How do they move and where?
Simple experience:
If you add half a glass of peas to half a glass of cereal without stirring, you get a whole glass. The grains and peas are small, but cannot “squeeze” into the empty spaces.
What if you pour equal amounts of water and alcohol into a beaker? Doubling the mixture, as in the first case, will not work. Why? The molecules of water and alcohol are completely different. There are greater distances between water molecules than between alcohol molecules. These gaps are partially filled, reducing the volume of the mixture.
In the same way, sugar molecules “scatter” throughout the entire volume, making the tea sweet.
(Sources and)
The penetration of molecules of one substance into the spaces between the molecules of another is called diffusion.
In gases, diffusion is also a fairly common process. Passing by a confectionery factory, people enjoy the smell of caramel, marshmallows, chocolate; entering a hairdresser, they smell the aroma of perfume, cologne, and eau de toilette. A person is constantly accompanied by smells. They carry important information about the substance due to diffusion. It proceeds faster in gases than in liquids, and here's why. Intermolecular distances in gases are greater than in liquids, hence the possibilities for movement are greater.
Is diffusion possible in solids? It must be taken into account that a solid body maintains its shape without changing, due to distances close to the dimensions of the molecules themselves or even smaller than them. Can “foreign” molecules penetrate there? It turns out that diffusion in solids is also possible, but only if they are in very close contact with each other. The process is very slow and time is measured in years. There is a known experiment with two polished bodies made of gold and lead. They were pressed tightly together and left under observation. Diffusion has occurred. Just 1 mm in five years.
()
The movement of molecules and the spaces between them explain diffusion. The hypothesis about this was first expressed by the Greek scientist Epicurus in the 3rd century BC.
This was experimentally proven in 1827 by Brown, the famous English botanist. He examined the spores of a moss plant in water through a microscope and noticed that these small particles were constantly moving in different directions. Non-stop, chaotic movement continued day and night, at any time of the year. Why did the plant spores move? And they moved because they were pushed and forced to move by water molecules invisible to a simple microscope.
Molecules move constantly, approach each other and, repelling, acquire a new direction of movement. There are many of them in matter, and meetings that change direction occur very often. The combined impact of several molecules sets into motion small particles that fall into the water. The movement of these particles was called Brownian motion, and the particles themselves were called Brownian.
()
So, you should remember:
- There are gaps between molecules
- Molecules move randomly and constantly
- The phenomenon of penetration of molecules of one substance into the spaces between the molecules of another is called diffusion.
Option 1
1. The smallest particles that make up various substances are called...
A. atoms B. molecules
2. All molecules of the same substance...
A. do not differ from each other B. differ from each other
3. When cooling, the volume of the body...
A. decreases B. increases
4. How does the diffusion process depend on temperature?
A. the diffusion process slows down with increasing temperature B. the diffusion process accelerates with increasing temperature C. the diffusion process does not depend on changes in temperature
5. At distances comparable to the sizes of the molecules (atoms) themselves...
A. the forces of attraction between molecules become more noticeable, and with further approach - the forces of repulsion B. the forces of repulsion between molecules become more noticeable, and with further approach - the forces of attraction
6. Which of the following properties belong to gases?
A. have their own shape B. retain volume C. do not have their own shape and constant volume
7. How are gas molecules arranged?
A. moving randomly in all directions, almost not attracted to each other B. do not diverge over long distances C. are located in a certain order
8. What state can mercury be in?
A. only in liquid B. in liquid, solid and gaseous C. only in solid
9. Is it possible to fill an open vessel with gas to 40% of its capacity?
A. yes, it is possible B. no, it is impossible C. a definite answer cannot be given
10. The water froze and turned into ice. Did the water molecules themselves change?
A. no, they have not changed B. yes, they have changed C. a definite answer cannot be given
Temperature
Due to the combinations of atoms in different substances, molecules are not the same. And if we consider one substance, but in different situations, for example, water from a tap, in a mug of tea or a spring stream. In all three cases the molecules are the same, but they behave differently. In hot tea, molecules move most quickly. It's much slower in a cold stream. And in tap water, molecules move slower than in hot tea, but faster than in a stream.
(Sources and)
Of course, we are talking here about speeds at which a person cannot move. A person can walk at a speed of 4 – 7 km/h. The speed of a water molecule at room temperature is on average 590 m/s = 2124 km/h (in boiling water - ≈ 2340 km/h) This must be understood when they say that some molecules move quickly, while others move slowly.
When they say “cold”, “warm”, “hot”, they compare the temperature. The temperature of any body is determined by how the molecules of this body move: fast or slow. The faster the molecules move, the higher the temperature. If the molecules begin to move more slowly, then the body temperature decreases.
The nature around us depends on temperature. With its decline comes autumn, and then winter. As temperatures rise, winter gives way to spring, and spring gives way to summer.
The properties of a substance also depend on temperature. For example, soft rubber becomes hard in the cold, and hard ice turns into liquid if it is brought into a warm room.
Temperature characterizes the properties of living and inanimate nature. This means there is a lot to know about her. The main thing to remember is this: the speed of movement of the molecules that form a substance determines its temperature.
To measure temperature, instruments called thermometers are used. Thermometers are designed for the following purposes:
- laboratory;
- medical;
- street;
- indoor;
- technical.
by design:
- liquid;
- gas;
- mechanical;
- electrical;
- optical.
Temperature is measured in degrees. In 1742, the Swedish geologist, meteorologist and astronomer Andres Celsius came up with a scale based on the reference points: the melting temperature of ice (0 degrees) and the boiling point of water (100 degrees).
()
In 1848, William Thomson (Lord Kelvin) introduced the concept of absolute zero temperature (-273 degrees, the lowest possible temperature in the Universe). At the same time, the melting temperature of ice is already 273 degrees and, accordingly, the boiling point of water is 373 degrees.
()
In 1724, the Polish-German scientist Daniel Gabriel Fahrenheit created his own scale, which was used in English-speaking countries. Currently it is only used in the USA.
()
On all three scales, two main points are used - the temperature of transition of the selected substance from one state to another.
On the Celsius and Kelvin scales, water is chosen as this substance, and the two points are the melting temperature of ice and the boiling point of water. The interval between these temperatures is divided into 100 parts, thus obtaining 1 degree.
Celsius chose the melting point of ice as 0°. Kelvin designated zero as the minimum possible temperature (when the movement of molecules ceases). Then, in Celsius, ice melts at 0° C (in Kelvin at - 273° K), water boils at 100° C, and in Kelvin at 373° K, since zero Kelvin is 273° below zero Celsius. When moving from degrees Celsius to degrees Kelvin, you need to add 273 degrees.
Fahrenheit called the main points a temperature close to the freezing point of mercury and the normal temperature of the human body. As a result, the melting temperature of Fahrenheit ice is about 32 degrees Celsius higher.
An example of transition from one scale to another:
normal room temperature
- on the Celsius scale – 20o C;
- on the Kelvin scale – оС + 273о = 20о С + 273о = 293о K;
- on the Fahrenheit scale – o С ∙ 9/5 + 32о = 20о С ∙ 9/5 + 32о = 68о F.
The Celsius scale is considered the most rational and easiest to use.
(Temperature is studied in detail in high school in the sections “Thermal Phenomena” and “Thermodynamics”).
Molecules move constantly and chaotically under any conditions. Movement affects temperature, which is why it is called thermal. Thermal motion is also transferred to Brownian particles. Definition of this phenomenon: the movement of solid particles trapped in a liquid under the influence of thermal motion of liquid molecules is called Brownian motion.
So:
- The movement of molecules determines body temperature
- Temperature is measured with a thermometer on the Celsius, Kelvin, Fahrenheit scales
- Temperature is a physical characteristic of the body
Summary of a physics lesson in 7th grade on the topic: “Structure of matter. Molecules.”
Physics lesson on the topic “Structure of matter. Molecules". 7th grade
Goals:
· Educational: introduce students to the structure of matter. Give an idea of the size of molecules.
· Developmental: develop logical and imaginative thinking.
· Educating: to cultivate hard work, a sense of responsibility and discipline during the learning process
Lesson objectives:
· introduce the concept of “atom” and “molecule” as models of the structure of matter;
· highlight experimental research in the process of cognition;
· emphasize the importance of modeling matter in the cognition of phenomena in the surrounding world;
· developing the ability to draw conclusions from observations;
· to form students’ desire to understand natural phenomena
Equipment: a tripod with a ring, a copper or brass ball that passes through the ring of the tripod, a flask, a glass tube, an alcohol lamp, potassium permanganate.
DURING THE CLASSES
1. Organizational moment
Checking absentees, the external condition of the office, workplaces, the presence of duty officers.
2. Updating knowledge
1) What does it mean to measure a quantity?
Answer: to measure a quantity means to compare it with a homogeneous quantity taken as a unit.
2) How is the scale division price of a measuring device determined?
Answer: in order to determine the price of a scale division, you must:
– find the two closest lines of the scale, next to which the value values are written; – subtract the smaller value from the larger value and divide the resulting number by the number of divisions between them.
3) Why does everyone need to know physics? Answer:
1) physics explains the causes of various phenomena, 2) allows you to create new, more and more advanced technology; 3) provides knowledge about the most general laws of nature that play a large role in the life of every person.
3. Study of new material “Structure of matter. Molecules"
Teacher: Guys, we have become acquainted with the concept of substance. Who remembers what is called a substance?
Student: Everything that physical bodies are made of is called matter. Substance is a type of matter, and matter is everything that exists in the Universe independently of us.
Teacher: Correct! Today we will find out what the substance consists of. Everything around a person - water, air, mountains, trees - have their own properties. Even in ancient times, 2500 years ago, some scientists suggested the structure of matter.
The Greek scientist Democritus (460-370 BC) believed that all substances consist of tiny particles.
This idea turned into a scientific theory only in the 18th century. and was further developed in the 19th century.
The emergence of ideas about the structure of matter made it possible to explain many phenomena and predict how they would occur under certain conditions. It became possible to influence the occurrence of phenomena. Many experiments confirm ideas about the structure of matter. Let's look at some of them. Let's try to squeeze a tennis ball. In this case, the volume of air that filled the ball will decrease. You can reduce the volume of an inflatable ball and a piece of wax if you apply some effort. The volume of the body also changes when heated. Let's do an experiment.
Demonstration of experience: take a copper or brass ball that passes through the tripod ring. Let's heat the ball and see what happens to it. If the ball is heated, it will expand and will no longer pass through the ring. After some time, the ball, having cooled, will decrease in volume, and the ring, heating up from the ball, will expand, and the ball will again pass through the ring.
Second experiment: Close the flask filled to the top with water tightly with a stopper. Pass a glass tube through the cork. The water will partially fill the tube. Note the liquid level in the tube. As we heat the flask, we will notice that after some time the water level in the tube rises.
Figure No. 2
Consequently, when heated, the volume of a body increases, and when cooled, it decreases. Now let’s divide into groups and each group will conduct experiments independently and at the end will make an experimental conclusion.
4. Group work
Card No. 1.
Equipment and materials: chalk, a bottle with crystals of potassium permanganate, 3 glasses of clean water, a glass rod.
Progress
1. Run your finger over the surface of the chalk. What are you observing? What can you say about the size of the particles that make up chalk? 2. Throw a few grains of potassium permanganate into a glass of clean water. Stir the solution with a stick and pour a few drops into a second glass, then repeat this procedure again. Compare the color of the solution in all three glasses. 3. Answer the questions:
Figure No. 3
– Is the main property of the substance – color – preserved? – Can you make an assumption about how many particles of potassium permanganate are left in the third glass? How many were there in the first glass then? – Remembering the size of the crystals you threw into the water, can you say anything about the size of the smallest particles of the substance?
5. Discussion of group work and putting forward a hypothesis about the structure of matter
· The volume of a body increases when heated, and decreases when cooled.
· All substances consist of individual particles, between which there are spaces.
· If particles move away from each other, the volume of the body increases.
· When particles come closer together, the volume of the body decreases.
· If all bodies consist of tiny particles, why do they seem solid to us?
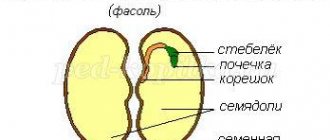
A molecule of a substance is the smallest particle of a given substance.
1. Using an electron microscope, we were able to photograph the arrangement of protein molecules. 2. Molecules of different substances differ from each other, but molecules of the same substance are the same.
Figure No. 4
Molecules are made up of small particles called atoms
Figure No. 5
1. Atoms are the particles that make up molecules.
2. Atoms are usually designated by special symbols.
3. O – oxygen atom
4. H – hydrogen atom
5. C – carbon atom
6. Consolidation
When we have put forward a hypothesis, we need to test it, try to explain various facts with its help. Let's solve a few problems. 1. The hand of the golden statue in the ancient Greek temple, which was kissed by parishioners, has noticeably lost weight over decades. The priests are in panic. Someone stole the gold! Or is this a miracle, a sign? Explain, based on Democritus’ hypothesis about the existence of the smallest particles of matter, what happened. 2. Wear and tear of shoes, depressions in the steps of ancient stairs, rubbing of the elbows of jackets, trousers... Don't these everyday phenomena lead to deep scientific reflections? Which ones? 3. You are doing your homework. From the kitchen comes the delicious smell of fried potatoes...How could this happen according to Democritus' hypothesis? Doesn't the spread of odors prove the existence of gaps between molecules?
7. Homework: paragraph 6, 7 (make an answer plan), ex. 2.
8. Summary
– What new did you learn from this lesson? That everything around us consists of molecules and atoms. How interesting is the science of physics, which introduces us to such observations, experiments and hypotheses, i.e. guesses about how the phenomenon occurs. And so the Greek scientist Democritus suggested that all substances consist of tiny particles, and this hypothesis was developed in the 19th century.
Interaction of molecules
All bodies are made of moving molecules. There are gaps between them. The question arises: why don’t bodies crumble into molecules or small crumbs? On the contrary, breaking a wooden stick requires a lot of effort.
()
The molecules are held tightly together. And if you try to connect two parts of a broken stick, it will not become whole. This means that there is a constant connection (interaction) between the molecules.
Bodies do not disintegrate into individual particles due to the attraction of molecules and atoms. On the other hand, if only intermolecular attraction existed, then there would be no gaps among the molecules, but they do exist. This means there must be repulsion. But it begins to manifest itself when the molecules come together at distances smaller than the size of the molecules themselves. The closer the molecules move, the more they repel.
In the case of a broken stick, the edges of the fragments have collapsed, and a person cannot simply, with just his hands, connect them so closely that the molecules begin to interact. Therefore, the result of the experiment will be zero.
And now another experiment. You need to separate two glass sheets lying on top of each other.
()
This is not easy to do. Why? Not hard to guess. The molecules of both sheets are quite close, since the sheets are very smooth. Interaction occurs (attraction of molecules).
A wet piece of paper is much more difficult to lift than a dry piece of paper from a polished table top. In a liquid, molecules are easier to move over distances when attraction begins to arise. If two separate droplets of water are connected, they will merge into one.
Mutual attraction of the liquid to the solid body is observed. It's called wetting. For example, water wets many fabrics, wood, paper, but is not attracted to plasticine, wax, or greasy surfaces. This process is called non-wetting. Wetting and non-wetting are found in everyday life, technology, living and inanimate nature.
In what cases will wetting be observed, and in what cases will there be non-wetting?
So:
- There is mutual attraction and repulsion between molecules
- The magnitude of attraction and repulsion is determined by the distance between the molecules
- Molecules and atoms of various substances can interact with each other
Basics
Three supporting facts constituted the initial information about the structure of matter. Let's list them:
- All bodies (gases, liquids, crystals, amorphous bodies) consist of molecules and atoms. Faraday later introduced the concept of ions - positively (cations) or negatively (anions) charged particles that can consist of one or more atoms.
- The particles that make up bodies have kinetic energy and are in a state of constant motion (for gases and liquids - chaotic movement, for solids - small vibrations).
- Particles interact with each other, attract and repel each other, and experience elastic collisions. Later, all interactions were considered electromagnetic.
Rice. 1. Brownian motion.
These ideas were arrived at not speculatively, but through experience. Brownian motion of suspended particles under the influence of molecular impacts, diffusion of gases and liquids, changes in the volume of substances as a result of heating or cooling, and so on. Initially, atoms were represented by some tiny particles, “balls”. The question of size and weight was resolved back in the mid-19th century, when the electron was discovered, a particle that was 1840 times lighter than hydrogen. But there was no idea about their structure one way or another. Despite this, Mendeleev managed to create a periodic table of elements and clarify the atomic masses of many substances (uranium, beryllium and others).
Faraday (explained electrolysis) and Maxwell (estimated the average range of gas molecules and laid the foundations of statistical mechanics) made a major contribution to the development of the theory.
They also knew about atoms that they could combine with each other. Two or more atoms combined into a common structure constitute a molecule. If the atoms are different, then the substance is called complex, if the atoms are the same - simple.
Rice. 2. Molecules of complex substances.
Based on these ideas, the differences in structure between solid, liquid and gaseous bodies were explained. Solids were described as ordered dense packs of oscillating atoms connected by a rigid bond (electrostatic interaction), and liquids and gases were described as a cloud of randomly moving particles (but in gases the distance between them is much greater than their own dimensions).
Rice. 3. Structure of solids.
Three states of aggregation
What substance is the most familiar and widespread on Earth? Of course, water. Water is found everywhere, and in different forms (ice or snow, liquid, steam). These are the aggregate states of water.
(Sources, and)
Absolutely all substances change their appearance and properties. This happens at a specific temperature for each substance. Oxygen, if cooled to – 183o C, becomes a blue liquid, and iron boils at 2750o C. It’s unusual, but it’s true.